Lyophilization (freeze-drying) is a common formulation technique applied to most classes of drugs, including small molecules and peptides. This article aims to start with the basics; what is the process, what are the advantages and disadvantages, and how is the drug administered after lyophilization.
What is lyophilization?
Also known as freeze drying, it is process of removing water under vacuum through sublimation. In addition to water, some solvents with high vapor pressure can also be removed, including tert-butanol & ethanol.
What is the starting point for lyophilization?
An aqueous, liquid formulation containing all the final components needed to deliver the drug; including the active pharmaceutical ingredient (API), together with the non-active components (called excipients). Typically, a specialized excipient called a cryoprotectant is added to a lyophilized product that would not be added to its liquid equivalent (more on cryoprotectants below).
What is the product of the lyophilization process?
The lyophilization process yields a glass vial containing a low density, solid powder, called a cake, typically white in color. The vial is sealed with a butyl rubber septum (stopper) and topped with a plastic/metal cap.

How do you administer a lyophilized drug product?
A sterile, aqueous diluent such as saline or water for injection, is added to the vial via a syringe punctured through the rubber septum. The vial is swirled, inverted, or shaken to help dissolve the lyophilized powder—this process is called reconstitution. Once fully dissolved, the liquid is withdrawn with a syringe and administered to a patient.
What is a cryoprotectant?
Addition of a cryoprotectant lowers the freezing point of water, helping to form smaller ice crystals which are less likely to mechanically damage the drug. Additionally, the cryoprotectant acts as a bulking agent to improve the physical integrity of the lyophilized cake and can improve reconstitution. Examples include mannitol, sucrose, and trehalose.
What is a lyophilization Cycle?
This is a series of distinct phases used to remove water. In each phase the temperature and vacuum are varied to fulfill specific roles during the lyophilization process.
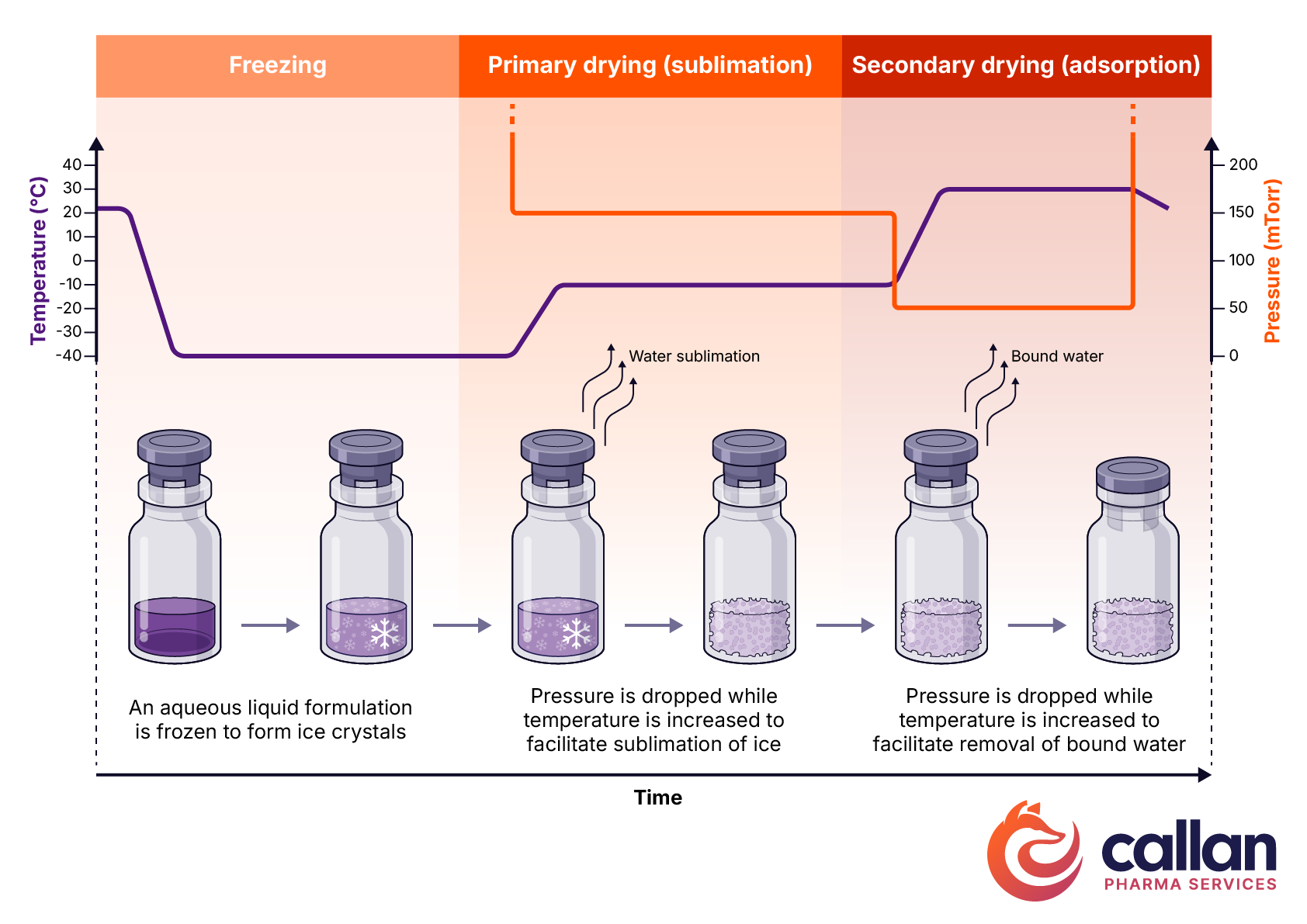
The 3 main phases of a lyophilization cycle are as follows:
- Freezing Phase: Freezing is necessary to form suitable ice crystals. This can be accomplished with a slower freezing process or through the incorporation of an annealing step in which the temperature is raised and then lowered.
- Primary Drying Stage (Sublimation) In this stage, the pressure is reduced, and the temperature is raised to facilitate sublimation of approximately 95% of the water as ice. This is the most critical stage for efficient lyophilization—striking the correct balance of pressure and the temperature is critical to effectively remove water.
- Secondary Drying Stage (Adsorption) The final stage involves further increasing the temperature above primary drying temperature and lower the pressure even further to remove water ionically-bound to the drug product.
What are the advantages of a lyophilized pharmaceutical?
The main advantages of lyophilization fall in the following 4 categories:
- Chemical stability – by removing water, you are removing one of main initiators of drug and excipient degradation. This can prolong the shelf-life and raise the storage temperature of a drug product.
- Storage temperature – lyophilized products are generally more stable at a wide range of temperatures compared to their liquid equivalents. This results in more economical storage and the ability to utilize the drug in remote locations that may lack cold infrastructure.
- Transportation – a lightweight dry powder is significantly more cost effective to transport than a heavy liquid formulation. Additionally, the added stability can eliminate the need to ship cold.
- Nanoparticle formation – during the slow and controlled lyophilization process, the removal of water (and in some cases co-solvent) can be used to encapsulate a drug in a nanoparticle structure. This offers a scalable alternative to nanoparticle forming techniques such as thin-film evaporation.
What are the disadvantages of a lyophilized pharmaceutical?
The disadvantages of lyophilization fall in the following 3 categories:
- Development – a lyophilization cycle should be develop specifically to meet the needs of a particular drug product. This adds time to development and narrows the list of laboratories who can help to those with lyophilization equipment and expertise.
- Manufacturing – lyophilization is an added step to the manufacture of a drug product which can add complexity, cost and time.
- Reconstitution – although the dissolving the lyophilized powder (reconstitution) is a straight-forward and simple process; it does add time making it less suitable for situations where a drug must be administered to a patient with short notice.
If you’re interested to learn if lyophilization is right for your drug, Callan Pharma Services can help with formulation and lyophilization development, including feasibility projects to get quick and cost-effective answers.


